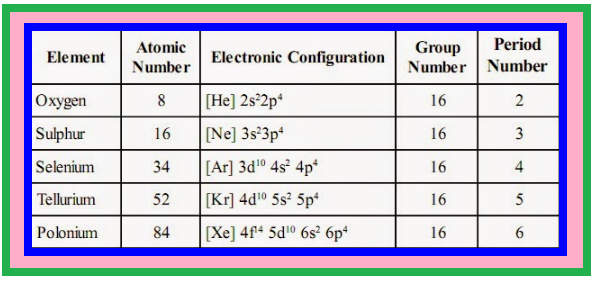
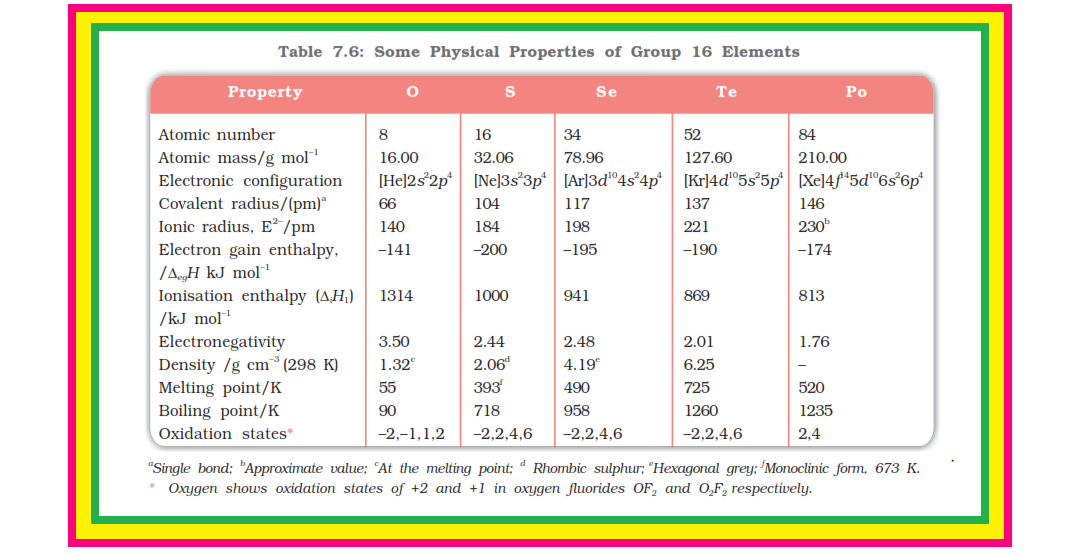
`=>` The anomalous behaviour of oxygen, like other members of `color{red}(p)`-block present in second period is due to its small size and high electronegativity.
`=>` One typical example of effects of small size and high electronegativity is the presence of strong hydrogen bonding in `color{red}(H_2O)` which is not found in `color{red}(H_2S)`.
`=>` The absence of `d` orbitals in oxygen limits its covalency to four and in practice, rarely exceeds two.
`=>` On the other hand, in case of other elements of the group, the valence shells can be expanded and covalence exceeds four.
(i) `color{green}("Reactivity with Hydrogen ")` All the elements of Group 16 form hydrides of the type `color{red}(H_2E (E = S, Se, Te, Po))`.
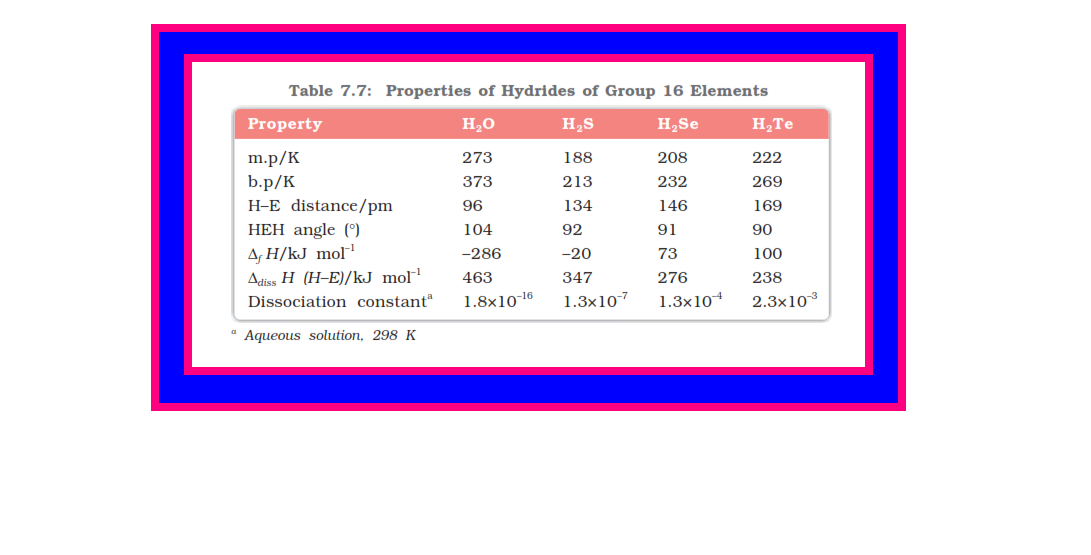
● Some properties of hydrides are given in Table 7.7.
● Their acidic character increases from `color{red}(H_2O)` to `color{red}(H_2Te)`. The increase in acidic character can be explained in terms of decrease in bond `color{red}(H–E)` dissociation enthalpy down the group.
● Owing to the decrease in bond `color{red}(H–E)` dissociation enthalpy down the group, the thermal stability of hydrides also decreases from `color{red}(H_2O)` to `color{red}(H_2Po)`.
● All the hydrides except water possess reducing property and this character increases from `color{red}(H_2S)` to `color{red}(H_2Te)`.
(ii) `color{green}("Reactivity with Oxygen ")` All these elements form oxides of the `color{red}(EO_2)` and `color{red}(EO_3)` types where `color{red}(E = S, Se, Te)` or `color{red}(Po)`.
● Ozone `color{red}(O_3)` and sulphur dioxide `color{red}(SO_2)` are gases while selenium dioxide `color{red}(SeO_2)` is solid.
● Reducing property of dioxide decreases from `color{red}(SO_2)` to `color{red}(TeO_2; SO_2)` is reducing while `color{red}(TeO_2)` is an oxidising agent.
● Besides `color{red}(EO_2)` type, sulphur, selenium and tellurium also form `color{red}(EO_3)` type oxides `color{red}(SO_3, SeO_3, TeO_3)`.
● Both types of oxides are acidic in nature.
(iii) `color{green}("Reactivity towards the Halogens ")` Elements of Group 16 form a large number of halides of the type, `color{red}(EX_6, EX_4)` and `color{red}(EX_2)` where `color{red}(E)` is an element of the group and `color{red}(X)` is a halogen.
● The stability of the halides decreases in the order `color{red}(F^(–) > Cl^(–) > Br^(–) > I^(–))`.
● Amongst hexahalides, hexafluorides are the only stable halides.
● All hexafluorides are gaseous in nature. They have octahedral structure.
● Sulphur hexafluoride, `color{red}(SF_6)` is exceptionally stable for steric reasons.
● Amongst tetrafluorides, `color{red}(SF_4)` is a gas, `color{red}(SeF_4)` a liquid and `color{red}(TeF_4)` a solid.
● These fluorides have `color{red}(sp^3d)` hybridisation and thus, have trigonal bipyramidal structures in which one of the equatorial positions is occupied by a lone pair of electrons. This geometry is also regarded as see-saw geometry.
● All elements except selenium form dichlorides and dibromides. These dihalides are formed by `color{red}(sp^3)` hybridisation and thus, have tetrahedral structure.
● The well known monohalides are dimeric in nature. Examples are `color{red}(S_2F_2, S_2Cl_2, S_2Br_2, Se_2Cl_2)` and `color{red}(Se_2Br_2)`. These dimeric halides undergo disproportionation as given below:
`color{red}(2Se_2Cl_2 → SeCl_4 + 3Se)`
`=>` The anomalous behaviour of oxygen, like other members of `color{red}(p)`-block present in second period is due to its small size and high electronegativity.
`=>` One typical example of effects of small size and high electronegativity is the presence of strong hydrogen bonding in `color{red}(H_2O)` which is not found in `color{red}(H_2S)`.
`=>` The absence of `d` orbitals in oxygen limits its covalency to four and in practice, rarely exceeds two.
`=>` On the other hand, in case of other elements of the group, the valence shells can be expanded and covalence exceeds four.
(i) `color{green}("Reactivity with Hydrogen ")` All the elements of Group 16 form hydrides of the type `color{red}(H_2E (E = S, Se, Te, Po))`.
● Some properties of hydrides are given in Table 7.7.
● Their acidic character increases from `color{red}(H_2O)` to `color{red}(H_2Te)`. The increase in acidic character can be explained in terms of decrease in bond `color{red}(H–E)` dissociation enthalpy down the group.
● Owing to the decrease in bond `color{red}(H–E)` dissociation enthalpy down the group, the thermal stability of hydrides also decreases from `color{red}(H_2O)` to `color{red}(H_2Po)`.
● All the hydrides except water possess reducing property and this character increases from `color{red}(H_2S)` to `color{red}(H_2Te)`.
(ii) `color{green}("Reactivity with Oxygen ")` All these elements form oxides of the `color{red}(EO_2)` and `color{red}(EO_3)` types where `color{red}(E = S, Se, Te)` or `color{red}(Po)`.
● Ozone `color{red}(O_3)` and sulphur dioxide `color{red}(SO_2)` are gases while selenium dioxide `color{red}(SeO_2)` is solid.
● Reducing property of dioxide decreases from `color{red}(SO_2)` to `color{red}(TeO_2; SO_2)` is reducing while `color{red}(TeO_2)` is an oxidising agent.
● Besides `color{red}(EO_2)` type, sulphur, selenium and tellurium also form `color{red}(EO_3)` type oxides `color{red}(SO_3, SeO_3, TeO_3)`.
● Both types of oxides are acidic in nature.
(iii) `color{green}("Reactivity towards the Halogens ")` Elements of Group 16 form a large number of halides of the type, `color{red}(EX_6, EX_4)` and `color{red}(EX_2)` where `color{red}(E)` is an element of the group and `color{red}(X)` is a halogen.
● The stability of the halides decreases in the order `color{red}(F^(–) > Cl^(–) > Br^(–) > I^(–))`.
● Amongst hexahalides, hexafluorides are the only stable halides.
● All hexafluorides are gaseous in nature. They have octahedral structure.
● Sulphur hexafluoride, `color{red}(SF_6)` is exceptionally stable for steric reasons.
● Amongst tetrafluorides, `color{red}(SF_4)` is a gas, `color{red}(SeF_4)` a liquid and `color{red}(TeF_4)` a solid.
● These fluorides have `color{red}(sp^3d)` hybridisation and thus, have trigonal bipyramidal structures in which one of the equatorial positions is occupied by a lone pair of electrons. This geometry is also regarded as see-saw geometry.
● All elements except selenium form dichlorides and dibromides. These dihalides are formed by `color{red}(sp^3)` hybridisation and thus, have tetrahedral structure.
● The well known monohalides are dimeric in nature. Examples are `color{red}(S_2F_2, S_2Cl_2, S_2Br_2, Se_2Cl_2)` and `color{red}(Se_2Br_2)`. These dimeric halides undergo disproportionation as given below:
`color{red}(2Se_2Cl_2 → SeCl_4 + 3Se)`